The 1st Herbal Research Webinar Series 2026 organised by UBD Herbal Research Group was successfully conducted on 11th February 2026 with Associate Professor Dr. Liza Md Salleh as the guest speaker. She delivered a presentation entitled “GoSwiet Softgels (Swietenia macrophylla) Research Driven and Market Ready” outlining her research and commercialisation journey in developing Go-Swiet Soft Gel derived from Swietenia macrophylla (commonly known as “tunjuk langit”). The webinar attracted a total of 40 participants from academia, industry, and healthcare sectors, including various faculties of Universiti Brunei Darussalam (UBD), IBTE Agro-Technology and Applied Sciences Campus, Universiti Teknologi Malaysia (UTM), University of the Philippines Manila, Hazara University Mansehra, Pakistan, United Pharma Sdn. Bhd., Ministry of Health and the Health Promotion Centre (Brunei), reflecting strong multidisciplinary interest in herbal and natural products research.

The talk emphasised how academic research can be translated into a market-ready herbal product through sustained scientific validation, technological innovation, and strategic collaboration. She explained that the project originated from growing public health concerns, particularly the increasing prevalence of diabetes and the demand for natural alternatives to conventional therapies, which motivated her team to investigate the bioactive potential of S. macrophylla seeds.
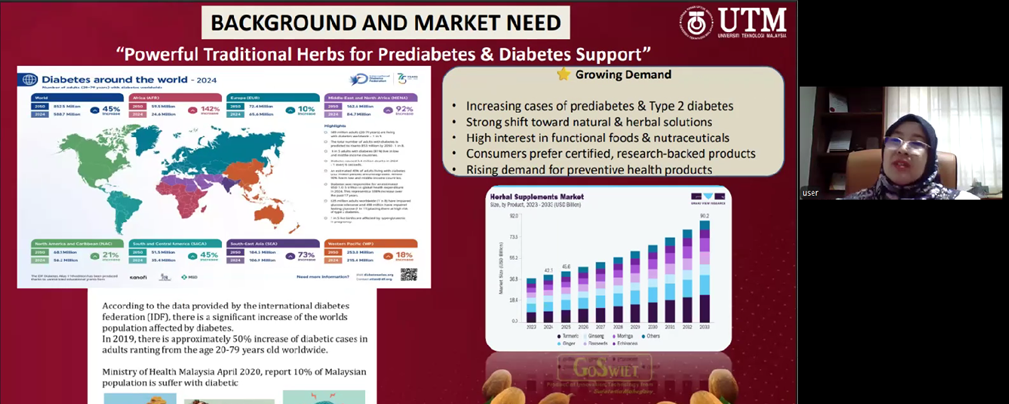
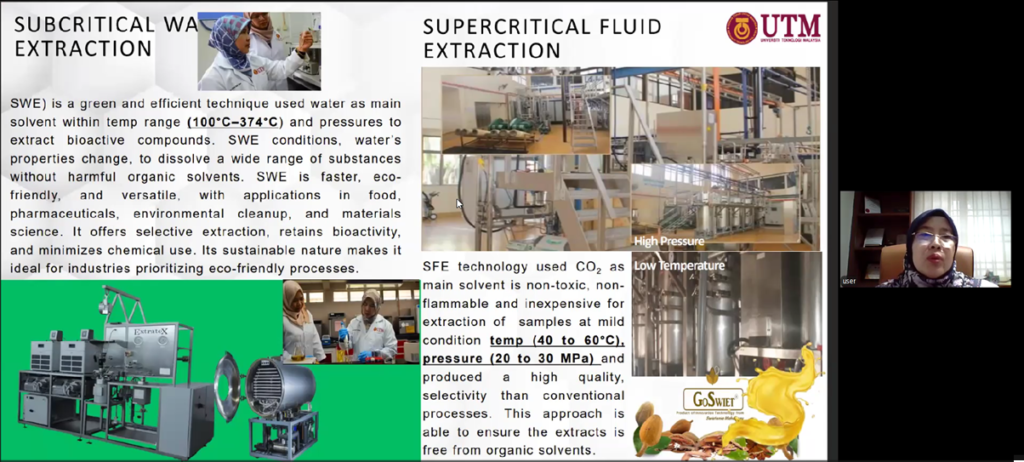
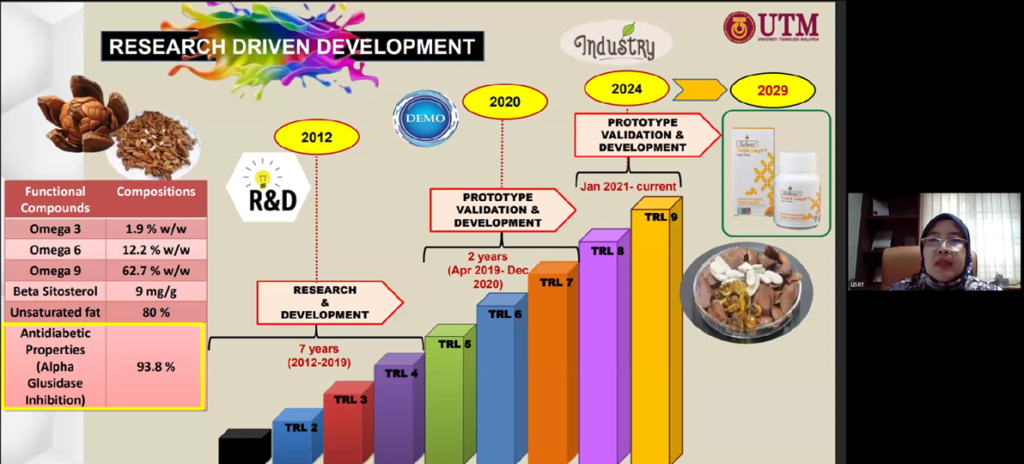
Dr. Liza described the scientific investigations undertaken to characterise the phytochemical composition of the seeds, identifying compounds such as flavonoids, saponins, and alkaloids that contribute to antioxidant activity and blood glucose regulation. To preserve these bioactive constituents, her team employed supercritical carbon dioxide (CO₂) extraction, an advanced technique that operates at high pressure but low temperature, producing a solvent-free, high-purity extract while maintaining compound stability. This technological approach enabled the transformation of a traditionally consumed but very bitter raw material into a standardised soft-gel formulation that is more convenient, palatable, and suitable for controlled daily dosage.
The presentation highlighted that the pathway from discovery to commercialisation required approximately seven years of intensive research and development, followed by additional years dedicated to prototype validation, regulatory approval, and manufacturing optimisation. Dr. Liza stressed that researchers must make a deliberate decision whether to focus solely on academic outputs such as theses and journal publications or to pursue the more demanding route of commercialisation, which involves intellectual property protection, product standardisation, and compliance with health authority regulations.
She further explained that successful translation required the formation of two complementary teams: a research and development group responsible for scientific validation and a business development team tasked with branding, promotion, and market engagement. Manufacturing was conducted through collaboration with GMP-certified facilities, while maintaining strict quality control, certificates of analysis, and regulatory compliance to ensure product safety and consistency. The product was positioned as a health supplement rather than a pharmaceutical replacement, reflecting regulatory frameworks that govern claims and usage.
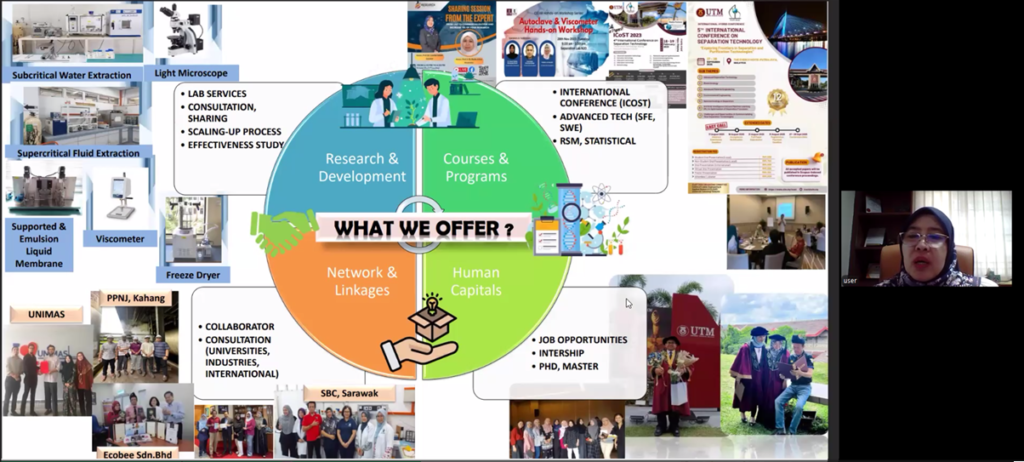
In concluding her talk, Dr. Liza underscored that universities play a critical role as innovation enablers by providing laboratory validation, technical expertise, and collaborative networks that help bridge the gap between traditional knowledge and modern evidence-based products. She encouraged researchers to build multidisciplinary partnerships, secure intellectual property early, and adopt scalable technologies to ensure that herbal research can generate tangible societal and economic impact beyond academia.