The Herbal Research Group, Universiti Brunei Darussalam, successfully hosted the 3rd Herbal Webinar Series on 2 July 2025, featuring Ms. Supaporn Intatham from Chiang Mai University, Thailand. Her talk focused on the anti-diabetic activities of medicinal plant recipes, showcasing a systematic approach to developing evidence-based herbal formulations.

In her presentation, Ms. Supaporn Intatham introduced her research on the anti-diabetic activities of medicinal plant recipes, conducted at the Clinical Research Centre for Food and Herbal Product Trial and Development, Chiang Mai University. She explained that the study was motivated by the need for safe, evidence-based herbal alternatives to support diabetes management, with a particular focus on type 2 diabetes and its underlying mechanisms, such as insulin resistance, oxidative stress, and pancreatic cell damage.

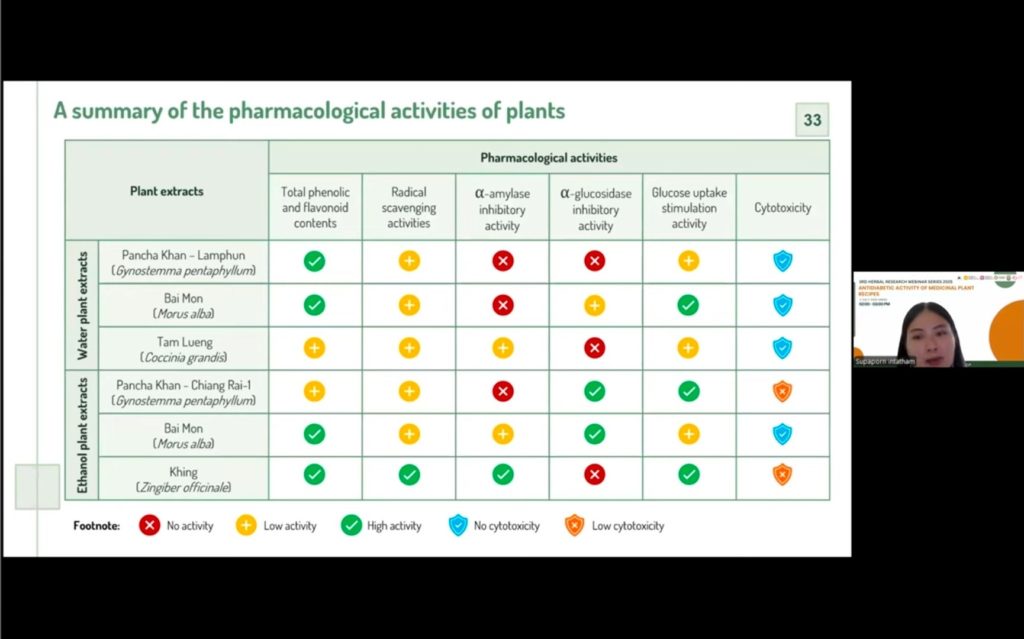
The research began with the selection and extraction of medicinal plants traditionally reported to have hypoglycemic properties. Seven plants were identified and sourced from different regions in northern Thailand, then prepared using both water and ethanol extraction methods. Their phytochemical profiles were examined to determine phenolic and flavonoid contents, followed by antioxidant testing using DPPH and ABTS assays. Results showed that plants with higher phenolic and flavonoid levels generally exhibited stronger radical scavenging activity.
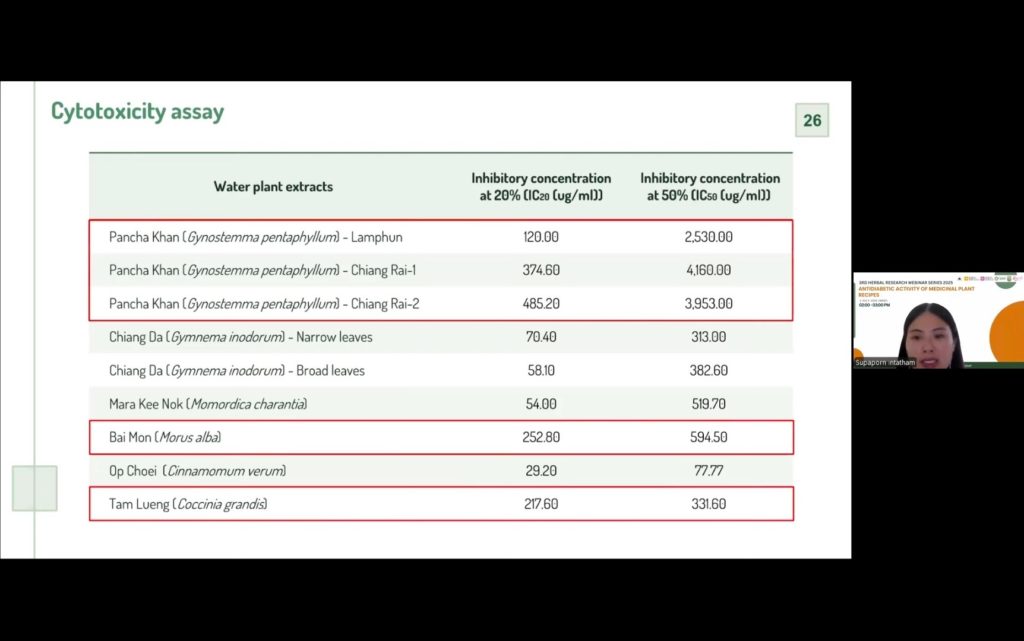
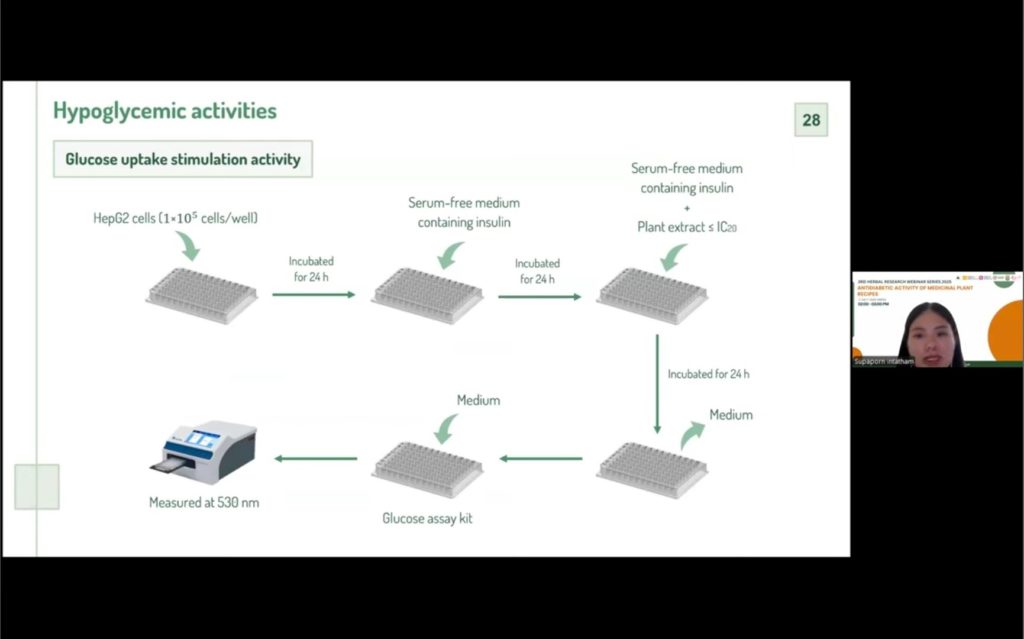
Subsequent hypoglycemic activity evaluations focused on enzyme inhibition assays, particularly α-amylase and α-glucosidase, which play critical roles in carbohydrate digestion and postprandial glucose levels. Some extracts demonstrated significant inhibitory activity, with several outperforming the standard drug acarbose. Additionally, cytotoxicity tests were performed to ensure the safety of candidate plants, with most extracts showing low toxicity, though a few were excluded from further studies. Glucose uptake assays in liver cells further highlighted extracts that could enhance cellular glucose absorption, an important mechanism for controlling blood sugar.
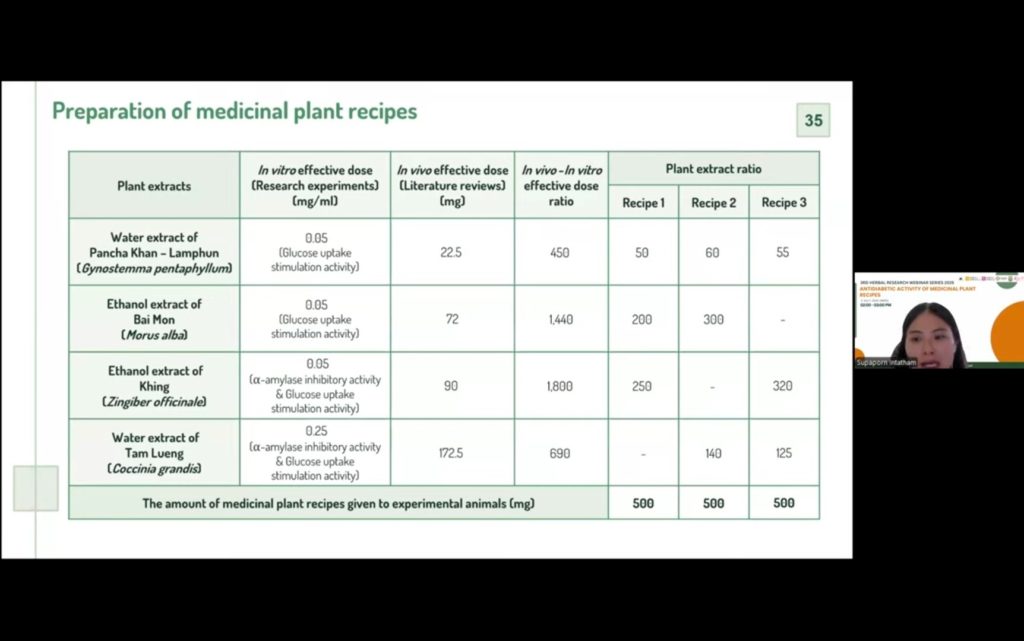
Based on these findings, four promising extracts were selected for recipe formulation: water extracts of Panchakan and Tam, and ethanol extracts of Bai Mon and Khing. The herbal recipes were developed by estimating optimal ratios from both in vitro and in vivo effective doses, resulting in three different formulations. These were tested again in vitro, showing varied mechanisms—two recipes inhibited α-glucosidase activity, while another enhanced glucose uptake in insulin-resistant cells.
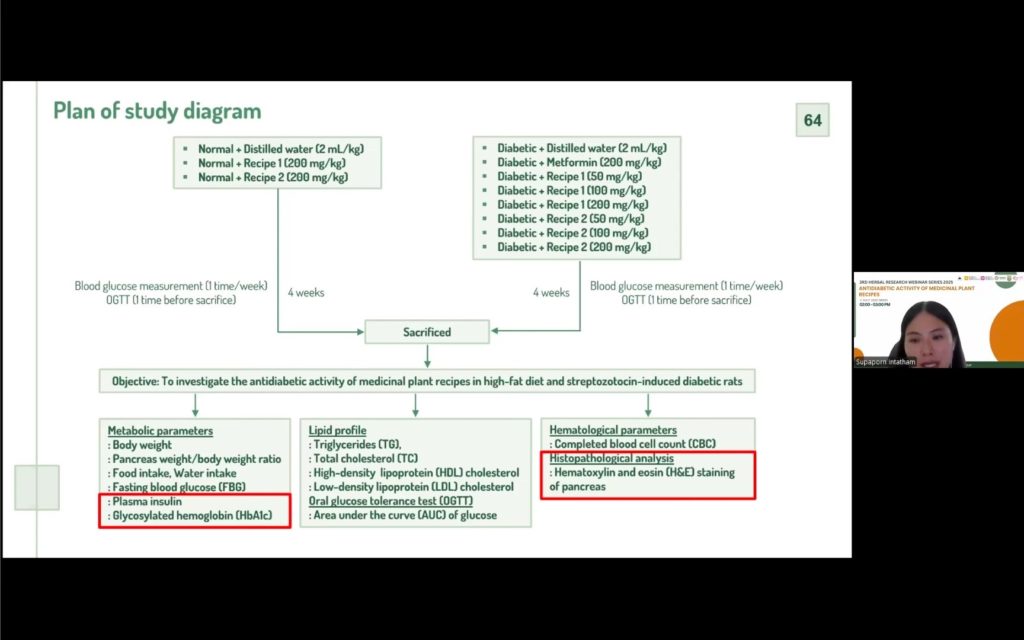
The study then progressed to in vivo testing using high-fat diet and streptozotocin-induced diabetic rats. Results indicated that two of the herbal recipes significantly reduced fasting blood glucose, improved body weight regulation, and positively influenced pancreatic health and lipid profiles, with effects comparable in some cases to metformin. Importantly, the recipes did not alter blood glucose in normal rats, suggesting that they are safe under non-diabetic conditions.
Ms. Supaporn emphasized that while the findings are still preliminary and ongoing, they strongly suggest the potential of these plant combinations as candidates for further development into herbal anti-diabetic products. She concluded by acknowledging her research team and supervisors, highlighting the importance of stepwise evidence generation before advancing into clinical trials.
During the Q&A session, participants raised questions about why three plants were chosen per recipe rather than more or fewer, the rationale for dosage ratios, and whether traditional ethnobotanical use influenced the formulations. Ms. Supaporn explained that recipe design was based primarily on in vitro and in vivo effectiveness, supported by literature evidence, rather than traditional practice, and that ratios were calculated using experimental dose-response data. Other questions covered cytotoxicity, extraction methods, and the safety of long-term use. She clarified that the extracts were prepared following Thai Herbal Pharmacopoeia standards and stored under controlled conditions, and that preliminary toxicity results indicated low risk for most plants. The session closed with interest in future collaborations, particularly in student training and joint research initiatives.